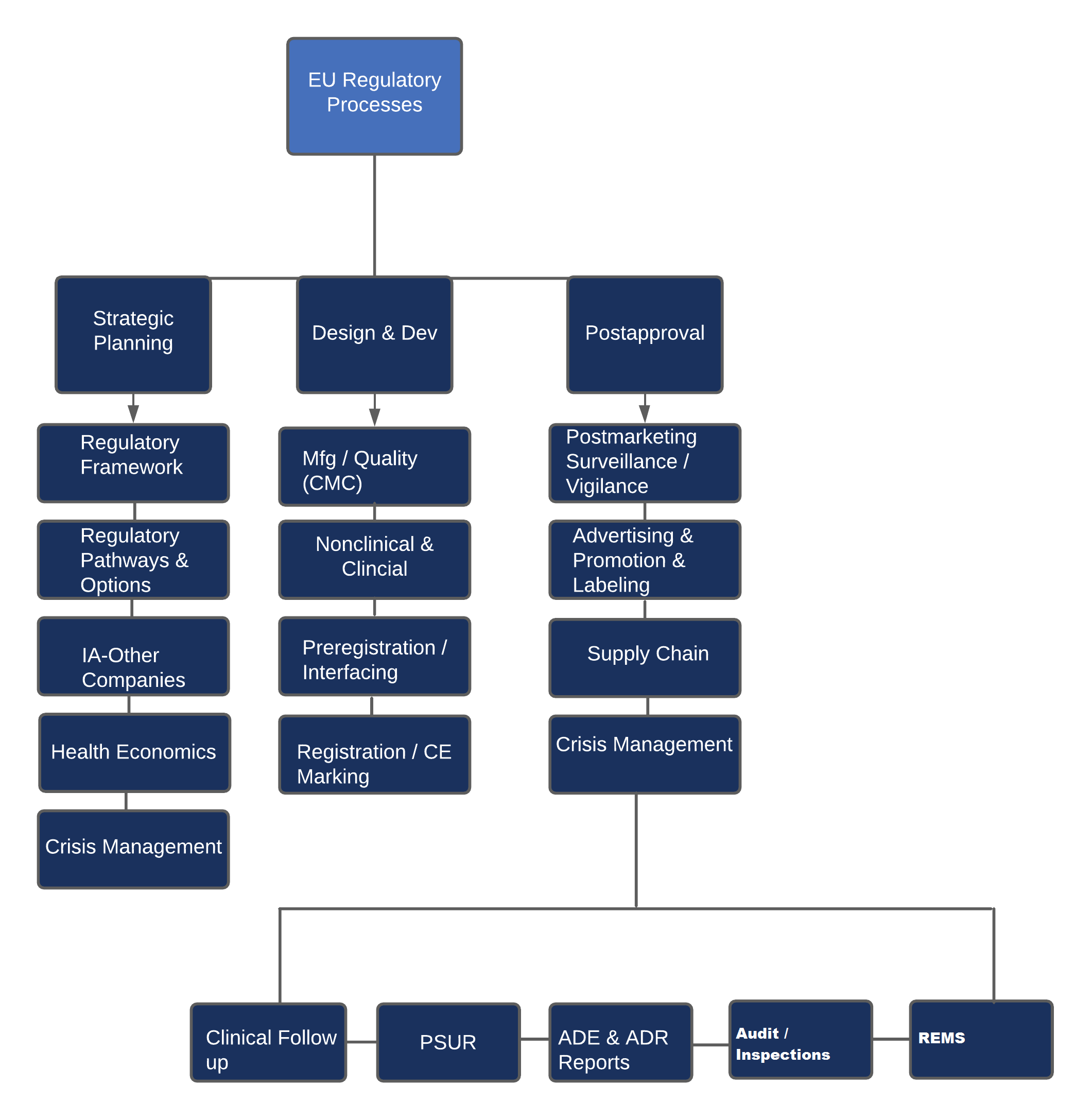
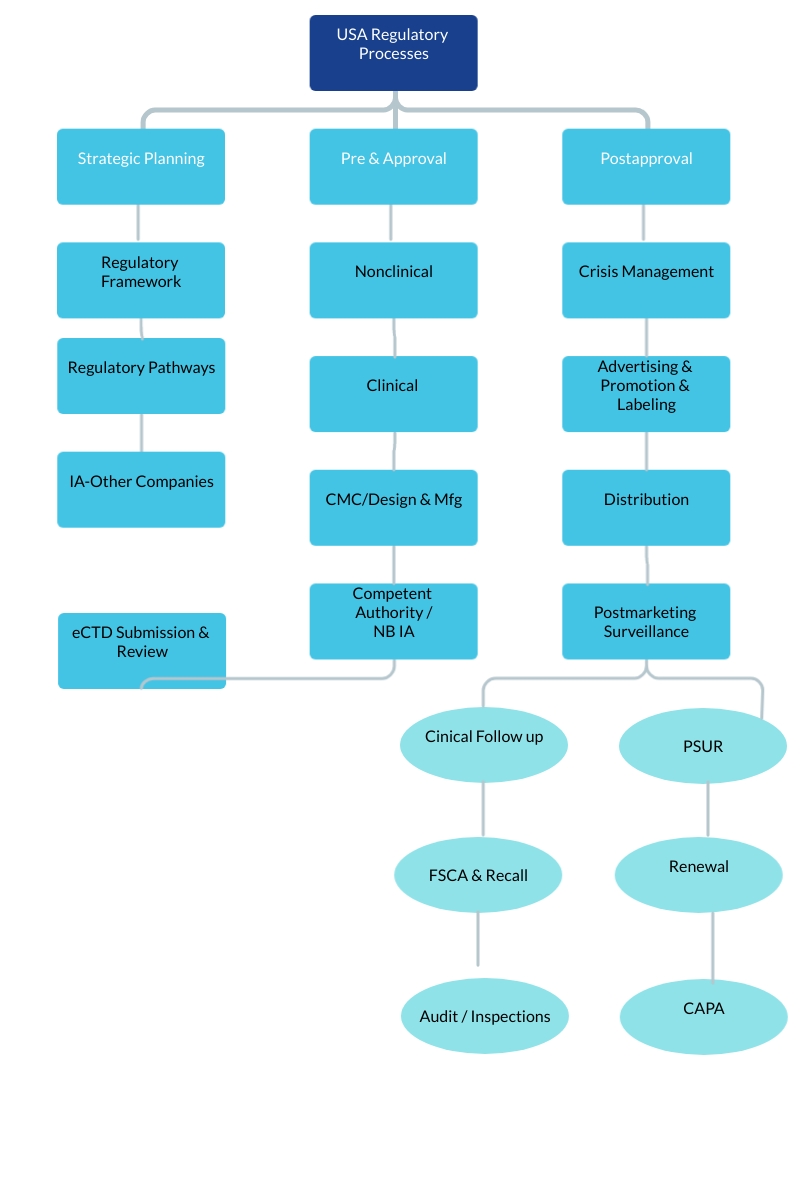

✿ Rest Of the World (ROW) Registrations
✿ Legalization across the world
✿ Notarization across the world
✿ Communication and Meetings with the Authorities
✿ Free Sales Certificate Import Licence
✿ No Objection Certificate
Type Certificate
✿ Other Registration requirements across the world
✿ The Certificate of Free Sale, also known as Free Sales Certificate, Certificate of Marketability, FSC or CFS can be ordered by you or by your EU Authorized Representative at the local competent authority upon request. A legalized Free Sales Certificate
acts as a proof of compliance with one of the most demanding regulatory systems in the world.
✿ It is also often a fast and cost-efficient way to sell your products in markets outside the European Union. We can help you to enter different markets worldwide – with Free Sales Certificates for your medical device or In vitro
diagnostic products! Many countries outside of the European Union without an own regulatory system accept the CE Mark for the import and sales of medical devices on their markets.
✿ Several countries are member of Apostelle Convention
✿ Several countries require only Notarization
✿ Many countries require Legalisation
✿ The procedures for Legalisation is different for each country.
✿ We can help you
to organize the necessary documents and order the Free Sales Certificate as well as taking care of the Legalisation